The Harm Reduction Race. Who's Winning?
The race is on for success in harm reduction: encouraging smokers to switch completely to reduced risk alternatives. Evidence of this rapidly evolving trend can be seen from all lanes — consumers, manufacturers, retailers, the government and even public health.
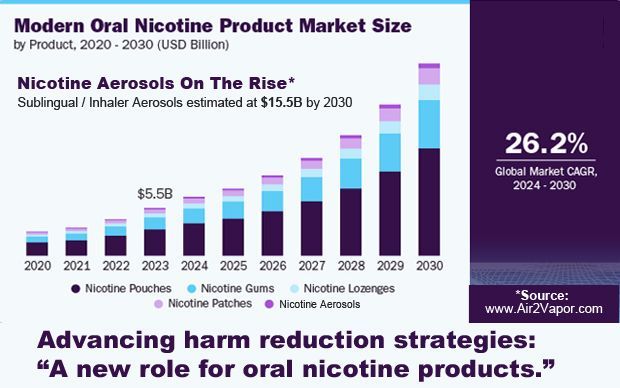
The widespread and growing availability of these products at retail has led to not only adult consumer awareness, but also interest and acceptance, as demonstrated by the rapid growth of the e-cigarette and modern oral categories in the United States. The modern oral (pouch) market has grown significantly over the past several years with some industry reports indicating a $1 billion increase in U.S. retail sales between 2022 and 2023.
The FDA authorizes reduced-risk alternatives via a limited number of premarket tobacco product application (PMTA) and modified risk tobacco product (MRTP) classifications. At Air2, we'd like to believe that the #FDA has not not given up on innovation and the science behind truly safer, reduce harm nicotine products, such as Air2's VaporEFX Inhaler and Sublingual. #VaporEFX
Altria’s Anderson agrees. To make true harm reduction a reality, he believes there needs to be faster progress from the FDA in three key areas:
- Authorizing smokefree products
- Informing adult smokers about the benefits of switching to smokefree alternatives
- Enforcing the law to eliminate illicit e-vapor products
“A strong course-correction is needed to protect the harm reduction opportunity for the 30 million adult smokers in the U.S.,” said Anderson, explaining that consumers are misinformed about the role of nicotine, for starters. “The FDA has authorized only a handful of smokefree products, woefully insufficient to meet the growing adult tobacco consumer demand. And the marketplace is being overrun by illicit disposable e-vapor products that are flouting FDA regulation and contributing to underage use.”
“Millions of adult smokers are seeking new options, including those that reduce risk, and their preferences are evolving rapidly,” Davien Anderson, a spokesperson for Altria, told Convenience Store News. “We believe it is our responsibility to create the conditions for harm reduction to succeed — through education, awareness and advocacy — as we build a strong portfolio of #smokefree products that satisfy adult smokers’ evolving interests and preferences. We know that millions of adult consumers 21-plus are interested in completely switching from cigarettes to a smokefree tobacco product.”
At Reynolds American, its vision of “Building a Smokeless World” means becoming a predominantly smokeless business with 50% of its revenue from noncombustible products by 2035. “We have already started our transformation journey and year by year, we will continue to actively encourage adult smokers who are uninterested in quitting tobacco products altogether to transition to smokeless alternatives,” a company spokesperson said.
The launch of heat-not-burn devices in the U.S. by PMI in October 2024, as well as the expanded consideration of other tobacco harm products internationally, will factor into the future success of harm reduction.
“These are important developments because every adult consumer has different needs and subjective preferences, and we must meet them where they are, both in their risk reduction journey and in the places and occasions that require this widened scope of products.